图1 腔镜下保留乳头乳晕乳房腺体切除加一期假体重建术手术步骤

Advances in Endoscopic Techniques for Surgical Management of Breast Cancer: A Comprehensive Review
- Journal of Sun Yat-sen University(Medical Sciences) Vol. 45, Issue 3, Pages: 361-369(2024)
DOI:10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).20240520.001
CLC: R737.9Published:20 May 2024,
Received:27 February 2024,
Accepted:14 May 2024
Scan for full text
Cite this article
PDF
Abstract
With the improvement in quality of life and the development of medical science and technology, people's requirements for postoperative beauty are correspondingly improved. On the premise of ensuring safety and feasibility, the advantages of endoscopic small incisions are used to treat breast diseases. With the vigorous promotion of scholars all over the world, endoscopic treatment of breast cancer has changed from concept to reality. This article will briefly describe the development history of endoscopic treatment of breast cancer, introduce the indications obtained by experts from all over the world through practice, as well as the surgical methods of endoscopic treatment of breast cancer that have evolved based on traditional breast cancer surgery, and discuss the controversy and prospect of endoscopic treatment of breast cancer.
Keywords
endoscope;
breast cancer;
surgery;
minimally invasive;
research development
全球范围内乳腺癌约占女性癌症的30%,其死亡率与发病率之比为15% [
1 腔镜治疗乳腺癌适应症进展
腔镜保乳术一般适用于早期乳腺癌患者,其适应症包括:①分期Tis,IA期-ⅡB期(T1-T2, N0-N2)患者;②肿瘤大小<3 cm;③影像学资料证实肿瘤未侵犯皮肤及胸大肌筋膜;④肿瘤距离乳头及乳晕≥2 cm。中华医学会发布的《乳腺癌腔镜治疗专家共识与操作指导意见(2019版)》[
2 腔镜治疗乳腺癌术式的拓展
随着腔镜治疗技术的进步,在确保安全性的情况下,腔镜下治疗乳腺癌术式得到拓展,主要手术方式主要包括腔镜下前哨淋巴结活检术、腔镜腋窝淋巴结清扫术、腔镜下保留乳头乳晕皮下腺体切除+I期假体植入术、腔镜下保留乳头乳晕皮下腺体切除自体组织移植乳房重建术。
2.1 腔镜腋窝淋巴结清扫术
乳腔镜腋窝淋巴结清扫术(mastoscopic axillary lymph node dissection,MALND),通过在腋窝附近区域利用三孔法对腋窝淋巴结进行清扫。有研究报道[
2.2 腔镜下保留乳头乳晕乳房切除加一期乳房重建术
腔镜下保留乳晕乳头乳房切除(endoscopic nipple sparing mastectomy,E-NSM)加一期乳房重建术(immediate breast reconstruction,IBR),在保留乳头-乳晕复合体功能的基础上行皮下腺体切除术。相对传统的改良根治术,在早期乳腺癌方面对患者的生存质量及心理负担有较好的改善。ENSM手术指征如上所述仅适用于早期乳腺癌且肿瘤不侵犯皮肤及胸大肌等患者,但也为早期乳腺癌患者提供一种新型的治疗方式,结合一期乳房重建术让患者的乳房更为接近原始状态,而一期乳房重建可以使用假体[
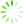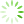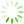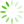
Fig. 1 The surgical steps for endoscopic assisted nipple-sparing mastectomy
A: Axillary incision for surgery; B: Excision of the blue-dyed sentinel lymph node through the axillary incision with intraoperative frozen pathology examination after injection of methylene blue tracer; C: Construction of a single-cavity space and detachment between the breast gland and pectoralis major muscle; D-E: Anterior and cross-sectional views demonstrate the radial injection of expansion fluid into the subcutaneous tissue of the entire breast through the areola; F-G: Anterior and cross-sectional views demonstrate the subcutaneous gland detachment through the axillary incision with scissors; H: Excision of tissue behind the nipple for frozen pathology examination; I-J: Anterior and cross-sectional views demonstrate the remaining glandular tissue around the breast under endoscopic guidance; K: Removal of glandular tissue, placement of encapsulated implants through the axillary incision into the chest muscle, and adjustment of implant position; L: Anterior view after breast surgery. [Drawing software: Paintwork (version 13.5.2_372) and WPS Office (version 12.9.1)].with direct-to-implant breast reconstruction
近期部分学者运用机器人辅助下保留乳头乳晕乳房切除,与E-NSM相比,避免了术中腔镜操作器械交织碰撞的机会并减少了对助手的依赖,但昂贵的器械成本限制其进一步推广。E-NSM术后的并发症主要是乳头乳晕坏死,有研究报道乳头部分坏死率为18%,全乳头坏死率为24%[
2.3 腔镜乳腺癌腋窝前哨淋巴结活检术
前哨淋巴结(sentinel lymph node, SLN)作为乳腺癌的第一个淋巴引流部位,代表了所有腋窝淋巴结(axillary lymph node, ALN)的状态。ALN受累是早期乳腺癌患者的重要预后因素,对肿瘤分期和临床决策具有重要价值[
2.4 腔镜下保留乳晕乳头皮下腺体切除的自体组织移植乳房重建术
目前医疗工作者正在努力寻找缩小乳房切口的方法,以减少术后瘢痕。对于小于整个乳房10% ~ 15%的小缺陷,采用与保乳手术相同的切口行腺体整形术,在乳房整体体积减小的情况下仍有望获得满意的美学外形。然而,对于构成整个乳房超过20%的中至大段缺损,自体组织移植是必要的。因此,可以根据缺损位置的特点选择各种重建方案[
目前自体血管重建的技术多种多样,应根据患者的解剖特点和术者的经验来选择。其中,局部皮瓣(侧胸背皮瓣或旋转皮瓣)是一种微型或保留肌肉的背阔肌(latissimus dorsi, LD)皮瓣,被认为是一种有用的修复外科方法,它可以应用于所有的乳房缺损,比扩大的LD皮瓣恢复更快。为尽量减少对功能性肌肉的划伤,也可采用肋间动脉穿支皮瓣或胸背动脉穿支(thoracodorsal artery perforator, TDAP)皮瓣等穿支皮瓣。然而,TDAP是一种可以选择性地用于除下内象限(lower inner quadrant, LIQ)以外的所有侧方位置的肿瘤的方法[
其次,乳房切除术中可能会损伤胸背血管。因此,由于肌肉萎缩和不确定的皮瓣活性,LD皮瓣若不可行可选择横行腹直肌(TRAM)皮瓣。此外,尽管有证据表明硅胶植入物是安全的,但一些患者不会接受,因此需要进行体积替代手术来达到对称。Hartrampf等[
美容性无瘢痕保留乳头乳晕的乳房切除术是乳腺癌手术中较为普遍的概念。虽然许多肿瘤外科医师在传统的保留乳头乳晕的乳房切除术中使用外侧切口或上外侧切口[
3 不同建腔方法的在乳腺癌腔镜微创手术的应用比较
3.1 溶脂法和非溶脂法
目前腔镜下乳腺癌切除术中分离皮肤与腺体过程中有分溶脂法与非溶脂法,溶脂法其目的是腔镜下减少脂肪对术程影响,以暴露韧带、腺体、血管、肌肉及神经等,从而方便术中解剖及分离,其过程是利用配置的溶脂液通过注射器分布于肿瘤周围脂肪,待一段时间后抽吸脂肪。此方法可获得较为清晰的解剖结构,但在肿瘤安全性方面仍有待考究。目前溶脂法主要用于腔镜下腋窝淋巴结清扫,杨絮等[
溶脂法与非溶脂法在腔镜下实施乳腺癌手术的应用是否会在术中引起肿瘤细胞的脱落种植转移成为争议的热点。溶脂法因抽吸脂肪获得操作空间及间隙显露清晰而被部分学者接纳,但其术中发生肿瘤细胞脱落转移的可能性使该使术者在开展时会产生顾虑。且溶脂法不适用于肥胖病人[
3.2 热刀法和冷刀法
在众多手术器械中,人们有时把它们分为热刀(热技术)和冷刀(冷技术)两大类。“热刀”指的是利用电流及其换能后的形式所产生的热作用而工作的技术,包括基础电刀、双极电刀、氩气刀、大血管闭合系统、超声刀、超声乳化吸引刀、射频刀等;“冷刀”指的是利用水流或制冷气体所产生的冷作用而工作的技术,包括水刀、冷冻器械等。基础电刀是最常用的热刀技术之一,通过高频电流在组织中产生热能,实现切割和凝固的功能。它具有精确控制切割深度和出血量的优势。超声刀(Harmonic Scalpel)利用超声振动来切割和凝固组织,它由一个产生超声能量并将其转换为机械振动的手柄组成。这种振动使组织蛋白质变性,从而实现切割和凝固。超声刀提供了切割和凝固功能的组合,能够在最小化出血的同时实现精确的解剖,在乳腺癌腔镜手术应用较为广泛,其在术中出血、术后引流情况及术后疼痛均优于使用传统电刀的患者,但超声刀的使用增加了手术的费用[
4 机器人手术在乳腺癌腔镜微创手术中的应用
2015年Toesca等[
目前,机器人辅助乳腺切除手术的适应症仍在探索,来自中国台湾、美国、韩国、意大利、法国等腔镜及机器人乳腺切除专家组发表了机器人乳房切除术共识[
5 展 望
在保证肿瘤安全性的前提下,腔镜技术的运用致力于使早期乳腺癌患者获得更高的生存质量及获得更好的乳房外观,有利于乳腺癌患者的身心康复。但从传统的开放手术跨越到腔镜下操作,手术时长的增加、对腔镜器械的使用、手术入路的选择等将对术者不断提出挑战。手术指征的严格把控也将影响乳腺癌患者的无瘤生存率。经过20余年中外学者的不懈努力,腔镜下治疗乳腺癌已成为被接受的术式,但更为规范、便于推广的手术入路仍在探索。不同于腹腔,需自造操作腔隙的乳腺使术中辅助器械变得尤为重要。希望借助科技的力量,让腔镜在治疗乳腺癌的道路上更上一层楼。
参考文献
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(1): 7-30. [Baidu Scholar]
Hinton CP, Doyle PJ, Blamey RW, et al. Subcutaneous mastectomy for primary operable breast cancer[J]. Br J Surg, 1984, 71(6): 469-472. [Baidu Scholar]
Iino Y, Ishikita T, Takeo T, et al. Subcutaneous mastectomy with axillary dissection for early breast cancer[J]. Anticancer Res, 1993, 13(4): 1183-1186. [Baidu Scholar]
Kroll SS, Ames F, Singletary SE, et al. The oncologic risks of skin preservation at mastectomy when combined with immediate reconstruction of the breast[J]. Surg Gynecol Obstet, 1991, 172(1): 17-20. [Baidu Scholar]
Palmer BV, Mannur KR, Ross WB. Subcutaneous mastectomy with immediate reconstruction as treatment for early breast cancer[J]. Br J Surg, 1992, 79(12): 1309-1311. [Baidu Scholar]
Ward DC, Edwards MH. Early results of subcutaneous mastectomy with immediate silicone prosthetic implant for carcinoma of the breast[J]. Br J Surg, 1983, 70(11): 651-653. [Baidu Scholar]
Lai HW, Mok CW, Chang YT, et al. Endoscopic assisted breast conserving surgery for breast cancer: Clinical outcome, learning curve, and patient reported aesthetic results from preliminary 100 procedures[J]. Eur J Surg Oncol, 2020, 46(8): 1446-1455. [Baidu Scholar]
中华医学会外科学分会乳腺外科学组, 姜军, 屈翔, 等. 乳腺癌腔镜治疗专家共识与操作指导意见(2019版)[J]. 中华外科杂志, 2020, 58(4): 4. [Baidu Scholar]
Breast Surgery Group of the Chinese Medical Association's Branch of Surgery, Jiang J, Qu X, et al. Consensus statements and operation guidelines on endoscopic surgery for breast cancer(2019)[J]. Chin J Surg, 2020, 58(4): 4. [Baidu Scholar]
Nakajima H, Fujiwara I, Mizuta N, et al. Video-assisted skin-sparing breast-conserving surgery for breast cancer and immediate reconstruction with autologous tissue[J]. Ann Surg, 2009, 249(1): 91-96. [Baidu Scholar]
Lai HW, Toesca A, Sarfati B, et al. Consensus statement on robotic mastectomy-expert panel from International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019[J]. Ann Surg, 2020, 271(6): 1005-1012. [Baidu Scholar]
徐洁, 陈曦, 夏苗火, 等. 非溶脂腔镜腋窝淋巴结清扫术在乳腺癌治疗中的临床研究[J]. 腹腔镜外科杂志, 2021, 26(4): 6. [Baidu Scholar]
Xu J,Chen X,Xia MH,et al.Clinical research of endoscopic axillary lymphadenectomy without liposuction in the treatment of breast cancer[J]. J Laparos Surg, 2021, 26(4): 6. [Baidu Scholar]
Xiong H, Chen Z, Xu L, et al. Contrast of mastoscopic and conventional axillary lymph node dissection of patients with breast cancer: meta-analysis[J]. Cancer Control, 2020, 27(2): 1073274820932987. [Baidu Scholar]
Luo C, Guo W, Yang J, et al. Comparison of mastoscopic and conventional axillary lymph node dissection in breast cancer: long-term results from a randomized, multicenter trial[J]. Mayo Clin Proc, 2012, 87(12): 1153-1161. [Baidu Scholar]
Ho WS, Ying SY, Chan AC. Endoscopic-assisted subcutaneous mastectomy and axillary dissection with immediate mammary prosthesis reconstruction for early breast cancer[J]. Surg Endosc, 2002, 16(2): 302-306. [Baidu Scholar]
Nakajima H, Sakaguchi K, Mizuta N, et al. Video-assisted total glandectomy and immediate reconstruction for breast cancer[J]. Biomed Pharmacother, 2002, 56 Suppl 1: 205s-208s. [Baidu Scholar]
Nakajima H, Fujiwara I, Mizuta N, et al. Video-assisted skin-sparing breast-conserving surgery for breast cancer and immediate reconstruction with autologous tissue: clinical outcomes[J]. Ann Surg Oncol, 2009, 16(7): 1982-1989. [Baidu Scholar]
Zhang S, Xie Y, Liang F, et al. Video-assisted transaxillary nipple-sparing mastectomy and immediate implant-based breast reconstruction: a novel and promising method[J]. Aesthetic Plast Surg, 2022, 46(1): 91-98. [Baidu Scholar]
Franceschini G, Visconti G, Garganese G, et al. Nipple-sparing mastectomy combined with endoscopic immediate reconstruction via axillary incision for breast cancer: a preliminary experience of an innovative technique[J]. Breast J, 2020, 26(2): 206-210. [Baidu Scholar]
Tukenmez M, Ozden BC, Agcaoglu O, et al. Videoendoscopic single-port nipple-sparing mastectomy and immediate reconstruction[J]. J Laparoendosc Adv Surg Tech A, 2014, 24(2): 77-82. [Baidu Scholar]
Lai HW, Chen ST, Mok CW, et al. Single-port 3-dimensional videoscope-assisted endoscopic nipple-sparing mastectomy in the management of breast cancer[J]. Plast Reconstr Surg Glob Open, 2019, 7(8): e2367. [Baidu Scholar]
Sakamoto N, Fukuma E, Higa K, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer[J]. Indian J Surg Oncol, 2010, 1(3): 232-239. [Baidu Scholar]
Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients[J]. Eur J Surg Oncol, 2008, 34(2): 143-148. [Baidu Scholar]
Ito K, Kanai T, Gomi K, et al. Endoscopic-assisted skin-sparing mastectomy combined with sentinel node biopsy[J]. ANZ J Surg, 2008, 78(10): 894-898. [Baidu Scholar]
Yamashita K, Shimizu K. Endoscopic video-assisted breast surgery: Procedures and short-term results[J]. J Nippon Med Sch, 2006, 73(4): 193-202. [Baidu Scholar]
Galimberti V, Vicini E, Corso G, et al. Nipple-sparing and skin-sparing mastectomy: review of aims, oncological safety and contraindications[J]. Breast, 2017, 34Suppl 1(Suppl 1): S82-s84. [Baidu Scholar]
Wang B, Ou C, Yu J, et al. Three-dimensional visual technique based on CT lymphography data combined with methylene blue in endoscopic sentinel lymph node biopsy for breast cancer[J]. Eur J Med Res, 2022, 27(1): 274. [Baidu Scholar]
Galimberti V, Cole BF, Viale G, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial[J]. Lancet Oncol, 2018, 19(10): 1385-1393. [Baidu Scholar]
Telli ML, Gradishar WJ, Ward JH. NCCN Guidelines updates: breast cancer[J]. J Natl Compr Canc Netw, 2019, 17(5.5): 552-555. [Baidu Scholar]
Goyal A, Newcombe RG, Chhabra A, et al. Factors affectingfailed localisation and false-negative rates of sentinel node biopsy in breast cancer–results of the ALMANAC validation phase[J]. Breast Cancer Res Treat, 2006, 99: 203-208. [Baidu Scholar]
White Jr RL, Wilke LG. Update on the NSABP and ACOSOG breast cancersentinel node trials[J]. Am Surg, 2004, 70: 420-404. [Baidu Scholar]
Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase Ⅲ trial[J]. Lancet Oncol, 2007, 8: 881-888. [Baidu Scholar]
Yao CC, Liu C, Xian J. Comparison of single-pore non-liposuction near-infrared laparoscopy with conventional open surgery for axillary sentinel lymph node biopsy in patients with early breast cancer: a single-center, small-sample retrospective study[J]. World J Surg Oncol, 2023, 21(1): 66. [Baidu Scholar]
Yang JD, Lee JW, Cho YK, et al. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (part 1): volume displacement[J]. J Breast Cancer, 2012, 15(1): 1-6. [Baidu Scholar]
Yang JD, Lee JW, Cho YK, et al. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (part 2): volume replacement[J]. J Breast Cancer, 2012, 15(1): 7-14. [Baidu Scholar]
Hamdi M, Van Landuyt K, De Frene B, et al. The versatility of the inter-costal artery perforator (ICAP) flaps[J]. J Plast Reconstr Aesthet Surg, 2006, 59(6): 644-652. [Baidu Scholar]
Santanelli F, Longo B, Germano S, et al. Total breast reconstruction using the thoracodorsal artery perforator flap without implant[J]. Plast Reconstr Surg, 2014, 133(2): 251-254. [Baidu Scholar]
Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap[J]. Plast Reconstr Surg, 1982, 69(2): 216-225. [Baidu Scholar]
Scheflan M, Dinner MI. The transverse abdominal island flap: Part I. Indications, contraindications, results, and complications[J]. Ann Plast Surg, 1983, 10(1): 24-35. [Baidu Scholar]
Spear SL, Carter ME, Schwarz K. Prophylactic mastectomy: Indications, options, and reconstructive alternatives[J]. Plast Reconstr Surg, 2005, 115(3): 891-909. [Baidu Scholar]
Spear SL, Hannan CM, Willey SC, et al. Nipple-sparing mastectomy[J]. Plast Reconstr Surg, 2009, 123(6): 1665-1673. [Baidu Scholar]
杨絮, 郭文斌, 甘霖霖, 等. 腔镜乳腺癌腋窝淋巴结清扫手术脱落细胞检查的初步研究[J]. 中国医师进修杂志, 2006, 29(12): 25-26. [Baidu Scholar]
Yang X, Guo WB, Gan LL, et al. Preliminary study of exfoliated cell examination in endoscopic axillary lymph node dissection for breast cancer[J]. Chin J Postgrad Med, 2006, 29(12): 25-26. [Baidu Scholar]
Brun JL, Belleannée G, Rousseau E, et al. Does axillary liposuction modify histologic study of excised lymph nodes?[J]. J Gynecol Obstet Biol Reprod (Paris), 1997, 26(5): 503-506. [Baidu Scholar]
Fang J, Ma L, Zhang YH, et al. Endoscopic sentinel lymph node biopsy and endoscopic axillary lymphadenectomy without liposuction in patients with early stage breast cancer[J]. Surg Oncol, 2017, 26(4): 338-344. [Baidu Scholar]
Wu QF, Yu YH, Zhu X, et al. Development of video-assisted breast cancer surgery: initial experience with a novel method for creating working space without prior liposuction[J]. Mol Clin Oncol, 2017, 7(1): 32-38. [Baidu Scholar]
Chengyu L, Jian Z, Xiaoxin J, et al. Experience of a large series of mastoscopic axillary lymph node dissection[J]. J Surg Oncol, 2008, 98(2): 89-93. [Baidu Scholar]
Kamprath S, Bechler J, Kühne-Heid R, et al. Endoscopic axillary lymphadenectomy without prior liposuction. Development of a technique and initial experience[J]. Surg Endosc, 1999, 13(12): 1226-1229. [Baidu Scholar]
Burdette TE, Kerrigan CL, Homa KA. Harmonic scalpel versus electrocautery in breast reduction surgery: a randomized controlled trial[J]. Plast Reconstr Surg, 2011, 128(4): 243e-249e. [Baidu Scholar]
Kerin MJ, O'hanlon DM, Kenny P, et al. Argon-enhanced cutting and coagulation confers advantages over conventional electrocautery for mastectomy[J]. Eur J Surg Oncol, 1996, 22(6): 571-573. [Baidu Scholar]
Bahls T, Frohlich F, Hellings A, et al. Extending the capability of using a waterjet in surgical interventions by the use of robotics[J]. IEEE Trans Biomed Eng, 2017, 64(2): 284-294. [Baidu Scholar]
Rau HG, Duessel AP, Wurzbacher S. The use of water-jet dissection in open and laparoscopic liver resection[J]. HPB (Oxford), 2008, 10(4): 275-280. [Baidu Scholar]
Keiner D, Gaab MR, Backhaus V, et al. Water jet dissection in neurosurgery: an update after 208 procedures with special reference to surgical technique and complications[J]. Neurosurgery, 2010, 67(2 Suppl Operative): 342-354. [Baidu Scholar]
Corvin S, Sturm W, Schlatter E, et al. Laparoscopic retroperitoneal lymph-node dissection with the waterjet is technically feasible and safe in testis-cancer patient[J]. J Endourol, 2005, 19(7): 823-826. [Baidu Scholar]
Wanner M, Jakob S, Schwarzl F, et al. Water jet dissection in fatty tissue[J]. Swiss Surg, 2001, 7(4): 173-179. [Baidu Scholar]
Toesca A, Peradze N, Galimberti V, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique[J]. Ann Surg, 2017, 266(2): e28-e30. [Baidu Scholar]
Gui Y, Chen Q, Li S, et al. Safety and feasibility of minimally invasive (laparoscopic/robotic-assisted) nipple-sparing mastectomy combined with prosthesis breast reconstruction in breast cancer: a single-center retrospective study[J]. Ann Surg Oncol, 2022. [Baidu Scholar]
Wu WP, Lai HW, Liao CY, et al. Use of magnetic resonance imaging for evaluating residual breast tissue after robotic-assisted nipple-sparing mastectomy in women with early breast cancer[J]. Korean J Radiol, 2023, 24(7): 640-646. [Baidu Scholar]
Angarita FA, Castelo M, Englesakis M, et al. Robot-assisted nipple-sparing mastectomy: systematic review[J]. Br J Surg, 2020, 107(12): 1580-1594. [Baidu Scholar]
Ryu JM, Lee J, Lee J, et al. Mastectomy with reconstruction including robotic endoscopic surgery (MARRES): a prospective cohort study of the Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG) and Korean Breast Cancer Study Group (KBCSG)[J]. BMC Cancer, 2023, 23(1): 571. [Baidu Scholar]
233
Views
419
Downloads
1
CSCD
Related Articles
Related Author
Related Institution

